What Does Cryogenic Processing Do?
Cryogenic Processing is not:
Cryogenic processing is not a coating. It affects the entire volume of the part. Cryogenic processing does not go away when the part is machined or sharpened. It affects every atom of the structure. This process works to enhance plating and many coatings
Cryogenic processing is not a substitute for heat-treating: It will not harden parts substantially. Used in conjunction with proper heat-treating, it produces a better, more stable part.
Cryogenic processing is not a fix for bad heat-treating: Although it will cause retained austenite to transform to martensite, so will cold treating at -140ºF. It will not cure the other ills of bad heat treat.
Cryogenic processing makes changes to the crystal structure of materials. The major results of these changes are to enhance the abrasion resistance and fatigue resistance of the materials. The changes we know about are:
- Change of Retained Austenite to Martensite in Hardened Steels.
- Reduction of Residual Stress.
- Precipitation of Fine Eta Carbides in Steel.
- Reduction in point defects.
- Re distribution of alloying elements.
- Making the crystal lattice structure more orderly.
The change of retained austenite to martensite happens in steel and cast iron only. It is the same with the precipitation of fine carbides. Many metallurgists will tell you outright that changing austenite to martensite is the only thing that cryogenic processing does. That is very wrong.
We know that there must be more than this. Why? Because cryogenic processing has been shown to work on materials other than steel. Brake rotors are made of cast iron that is pearlitic in structure. There is no retained austenite but scientific tests show an increase in life of up to seven times. Most metals will respond to cryogenic processing. Some plastics do. There is a lot of evidence that crystals such as diamonds, cubic boron nitride, and aluminum oxide also respond.
Some Basic Metallurgy
Metals are crystalline in structure. That means that the atoms of the metal line up in an orderly fashion. A theoretical crystal structure is pictured at the left. Notice how perfect it is. This rarely happens in the real world.
A real world crystal structure is pictured, bottom left. The yellow dots are the atoms. Note that the alignment of all the atoms is not perfect. There are discontinuities and changes in spacing. A theory proposed by Dr. Mark Eberhardt at the Colorado School of Mines states that there is a discreet distance between atoms where the energy in the metallic bond is minimum.
Picture below is courtesy of and used with the permission of Birmingham University’s Nonascale Physics Research Lab. We wish to thank Professor Richard Palmer for allowing us the use of the picture.
Their explanation for the picture is:
Copyright, Nanoscale Physics
Research Laboratory,
University of Birmingham
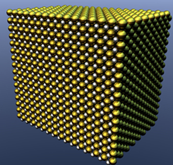
“Atomic Love, in three Dimensions
Made of pure palladium, is only 8 nanometers in size; you can even see the atoms. Clusters of palladium bonded together on the surface of carbon and spontaneously arranged themselves into the world’s smallest heart.”
We know that when we reduce the temperature of an object the atoms in the object come closer together and we take energy out of the object. We have a theory that when we warm the object back up that some of the misplaced atoms in the crystal structure relocate to the proper spacing. This produces a more perfect crystal we’ve discussed this theory with metallurgists and scientists. Nobody has said we are crazy yet.
Atoms in a crystal structure can line up in different formations. Most metals use one of three different formations, although other formations occasionally appear.
The three basic formations are:
- Body Centered Cubic
- Face Centered Cubic
- Hexagonal Close Packed
Austenite to Martensite Transformation
What are austenite and martensite?
What are the other things that happen?
We think that they are :
- Reduction of Vacancies in the Crystal Lattice.
- Refinement of the Atom to Atom Spacing.
- Re-Ordering of the Alloying Elements.
In general, the process seems to refine the crystal structure of metals and crystalline plastics. Although there has not been definitive research on the subject, the theory is that it crystal structures are not perfect. Some research shows that there are millions of vacancies per cubic inch of metal in the crystal lattice. Also, some atoms are not ideally located in respect to their nearest neighbors. We believe that cryogenic processing makes the crystal more perfect and therefore stronger.
We have some evidence from work that we did with Honeywell on thin film magnetic memory chips. They found that our process relieved stresses between the layers, increased conductivity, and also there was some evidence holes in these very thin films (on the order of several atoms thick) were gone after processing. This indicated a shifting of atoms in the crystal lattice. Another indication is that metal objects transmit sound or vibration differently after processing. We repeat that this is just theory and requires verification.
Change Retained Austenite to Martensite in Hardened Steels
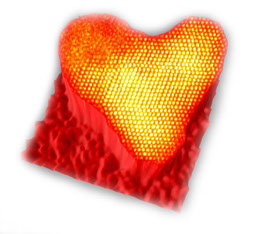
It has been known for many years that cold will cause retained austenite to change to martensite. (The terms austenite and martensite refer to the way the carbon atoms relate to the ferrous atoms in the crystal lattice structure. Note that we refer to a crystal lattice structure. A lot of people try to talk about the “molecular” structure of metals. Metals are metals because they are crystalline in nature. The crystal structure is what gives the metals their ability to conduct heat and electricity, their ability to plastically deform, and their ability to be hardened.) This can be verified through publications such as Machinery’s Handbook, ASM’s Metals Handbook and more.
Even the best heat treating facility will leave somewhere between ten and twenty percent retained austenite in ferrous metals. We’ve seen over 40% on gears and shafts made for commercial and racing applications. Because austenite and martensite have different size crystal structures, there will be stresses built in to the crystal structure where the two co-exist. Cryogenic processing eliminates these stresses by converting most of the retained austenite to martensite. This also creates a possible problem. If there is a lot of retained austenite in a part, the part will grow due to the transformation. This is because the austenitic crystals are about 4% smaller than the martensitic crystals due to their different crystal structure.
The process also promotes the precipitation of small carbide particles in tool steels and steels with proper alloying metals. A study in Romania found the process increased the countable small carbides from 33,000 per mm3 to 80,000 per mm3. The fine carbides act as hard areas with a low coefficient of friction in the metal that greatly adds to the wear resistance of the metals. A Japanese study (Role of Eta-carbide Precipitations in the Wear Resistance Improvements of Fe-12Cr-MO-V-1.4C Tool Steel by Cryogenic Treatment; Meng, Tagashira, et al, 1993) concludes the precipitation of fine carbides has more influence on the wear resistance increase than does the removal of the retained austenite.
The process relieves residual stresses in metals and plastics:
This has been borne out by several research projects as well as by practical use. We have customers who cryogenically treat metal before heat treat to reduce the distortion of the metal during heat treat. NASA is one of them. We processed components for the space shuttle’s robotic arm for this reason. Gage makers have used cold temperatures to stabilize metals for years. Our work with Honeywell also showed that Cryogenics Processing relieves stresses, as does a recent NASA study on welded aluminum.
For more on Cryogenic processing go to https://ctpcryogenics.com/cryogenics/what-is-cryogenic-processing/theory-of-cryogenics/